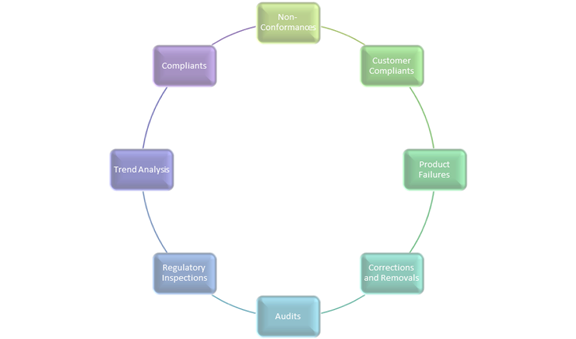
Corrective Action and Preventive Action (CAPA) is a subsystem that ensures that problems are detected and resolved. The sources for CAPA(s) arise from non-conformances, customer complaints, product failures, corrections and removals (product recalls), process deviations, audits, regulatory inspections, and trend analysis from process performance and product quality monitoring/ reviews boards.
At Biotech Solns Int'l we can help you develop a structured, methodical approach to the investigation process in the determining and correcting the root cause of the issue to prevent recurrence. Also, we can assist you in the development and implementation of a CAPA system that focuses on investigations that aligning with the level of risk associated with the issue.